CORONAFOCUS
Covid-19 PCR Day 2 and Day 8 Laboratory Travel Tests for Mandatory Government International Travel Testing
RESULTS IN < 24 HOURS FROM RETURN OF CORONAFOCUS
SELF-SAMPLING PCR KITS TO OUR LAB.
The first private testing lab to be listed as a private provider on the
Government GOV.UK website for day 2 and day 8 coronavirus international
travel arrivals.

Gold standard Covid-19 PCR testing and
sequencing performed at our Cambridge laboratories.
Capacity for 40,000 Coronavirus PCR tests daily.

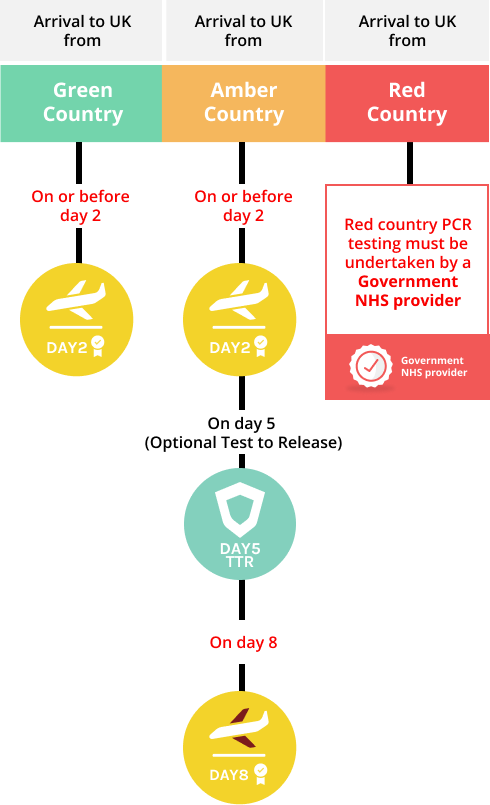
International arrivals. What PCR test should I take?
GREEN COUNTRIES
-

Fast, accurate results in < 24 hours from booking sample into lab
-

1 kit delivered free, for convenient home sampling
-

Easy to follow swabbing instructions
-

Free pre-paid return envelopes
-

Genomic sequencing of positive day 2 test (Sequencing not UKAS accredited – in progress)
-

Optional return swab drop-in to lab or via own private courier
-

Phone, Chat and email support
-

Issue of valid code for passenger locator forms
-

Result and certificate download from our online dashboard
-

Government approved testing and sequencing provider
AMBER COUNTRIES
-

Fast, accurate results in < 24 hours from booking sample into lab
-

2 kits delivered together – one day 2 and one day 8 test
-

Easy to follow swabbing instructions
-

Free pre-paid return envelopes
-

Genomic sequencing of positive day 2 test (Sequencing not UKAS accredited – in progress)
-

Optional return swab drop-in to lab or via own private courier
-

Phone, Chat and email support
-

Issue of valid code for passenger locator forms
-

Result and certificate download from our online dashboard
-

Government approved testing and sequencing provider

COVID-19 TEST TO RELEASE
COVID-19 SELF-SAMPLING TRAVEL
TESTING PACKAGE
-

Results in < 24 hours from booking in of sample at lab
-

Instant result and certificate download on our online dashboard
-

Issue of valid TTR code for passenger locator forms
-

Optional Swab drop-in to lab
-

Free pre-paid return labels
-

Easy to follow swabbing instructions
-

Results valid for release from quarantine (Test To Release Approved).
Coronavirus PCR
Swab Testing
The Covid-19 PCR Test journey through
our genomic testing laboratory.
A PROVEN TRACK RECORD IN ACCURATE TESTING
Leaders in accurate Covid-19 PCR testing and sequencing with capacity for 40,000 PCR
lab tests daily
How to take a
self-swab for PCR
COVID-19 Testing



Scientist's signature
Each certificates is signed by a registered clinical scientist in accordance with the regulatory authorities.

Clear Results
The certificate includes personal information, test results, date of sample receipt at the laboratory, the date the swab was taken.

Helpline and phone validation
There is a dedicated number for confirming the validity of the certificate by airport authorities.
Frequently Asked Questions to aid your travel testing journey
Travel planning with Coronofocus Covid test kits
- 2 self-sampling Coronafocus PCR tests each sent separately as per government rules for lab analysis on or before day 2 and day 8 of quarantine.
- Day 2 is sent out first. Day 8 is sent out on day 7 to arrive for self-swabbing on day 8. If day 8 falls on weekend your kit will be delivered on Monday. We cannot send both PCR test kits out together, as it is a legal requirement that they are sent separately.
- Your PCR positive/negative results are published within 24 hours of us receiving your sample at the lab and are viewable in the online customer dashboard.
- Genomic sequencing of results tested as positive on day 2 of quarantine are provided to NHS track and Trace for variant surveillance.
- If a positive result is received from the day 2 test, individuals will not be required to take a further test but will need to isolate for 10 days from the day of positive result notification.
- The kit includes a packaged self-sample swab, liquid tube, sealable bag and a set of instructions of how to complete the self-swab test correctly and prepaid envelope labels.
You can buy your Coronafocus PCR tests here up to 6 months before travelling. You will receive the test kit for day 2 between day 0 and day 2, the test kit for day 5 Test to Release (TTR) on day 5 and test kits for day 8 on day 8. Once a self-sampling Coronafocus swab has been taken, it must be returned by postal courier using the prepaid envelope or hand delivered.
If your flight asks for a test to be done 72 hours in advance, ensure you allow time for your test to be delivered.
Example: if flying on a Saturday, then we recommend ordering your test on our website latest on the Tuesday before noon GMT prior to date of travel. So in order to receive and take the self-sample swab for testing on the Wednesday prior to flying, this requires return dispatch to our lab using the prepaid envelope, so that our lab receives it on the Thursday prior to flight. Results would then be available for viewing on the online dashboard, details in confirmation email on the Friday before flight. We cannot guarantee that the swab will arrive the day after postage, as this depends on the Royal Mail service which can sometimes take longer than 24 hours. This is why we recommend travellers endeavour to purchase well in advance of travel.
If you are leaving the UK, then depending on the destination country entry rules you will need a Pre-Travel or Fit to Fly PCR test with a certificate of negative test result. If you are coming into the UK, we offer a Day 2 and Day 8 PCR Testing Package that is a government requirement. We also offer a Day 5 Test to Release kit that can release you from isolation early.
For children travelling abroad check entry requirements for your destination country. GOV.UK foreign travel advice.
For children returning to the UK under government guidelines:
- Children aged 10 and under returning from Amber/Green countries do not need to take a covid pre-departure test.
- Children aged 4 and under arriving from Amber/Green countries do not need to take day 2 or day 8 tests as per the rules here.
Self-Test Information
Our Covid-19 PCR test looks for the presence of the virus currently in the body, it detects virus RNA in the Coronafocus self-swab sample. We extract the RNA genetic material and copy it into DNA. Now if the virus is there, it is in a DNA form. We put it into a PCR reaction (Polymerase Chain Reaction) that amplifies snippets of DNA and matches these to the viral DNA sequence. The St Mary’s Covid-19 PCR test is very specific and accurate. If our test finds it, you can believe it.
Once the test has been purchased and dispatched to you, it is your responsibility to correctly handle the liquid tube avoiding spillage and undertake the self-swab correctly following the instructions provided. If in contact with an external surface, use ethanol-based decontamination and cleaning solutions. Do not use bleach or other disinfectants.
The swabs used in Coronafocus self-sample PCR kits are ETO sterilised (Ethylene Oxide).
Results Account and Receiving Results
Instructions sent on booking on the St Mary’s website explain how to register and activate your PCR test kit in the online customer dashboard using the unique password sent to the booking contact and email address.
- The date and time the swab is taken must be entered in the online customer dashboard to enable lab processing.
- You can search for “User registration” or “St Mary’s”, in your inbox or junk mail.
- Customers who have booked through a different provider will have been given a pin code by that provider, they should enter this to activate their kit.
- Activating your PCR test kit via mobile phone is not recommended, customers are advised to use google chrome on a laptop or desktop. The five boxes on the customer dashboard for activation should be filled with the date (day/month/year), and time (hour/minutes). This should be done in 24hr format.
To access your results in our customer dashboard you must register and activate your test kit in the online customer dashboard. We will not process your returned self-swab test until this is done. You must then enter the date and time swab taken in the 5 boxes in the online customer dashboard otherwise your sample will be placed on hold. Results are published in the online customer dashboard within 24 hours of St Mary’s receipt of activated date stamped self-sampled swab to our lab, for viewing or certificate download. If the test kit is sourced through a third party then login using the details provided by the company you booked through
Check you have put in all the correct details into the online St Mary’s Customer Dashboard. This needs to be done on a computer and not via a tablet or mobile. If not booked with St Mary’s, please refer to the Company you purchased from for login details.
PCR Test sample rejection may be due to a number of reasons, i.e. the sample provided was non-optimal to determine whether individuals are infected with the virus. This can be due to leakage or damage in transit providing insufficient sample volume for testing, labelling issues, stability time exceeded, expired tubes, or no sample received. Factors such as consuming foodstuff 30 minutes prior to performing the swab and using oral hygiene products are not recommended prior to swab. If notified that your PCR Test sample was rejected, we ship a replacement kit and advise you to swab as soon as possible and return the sample.
Additional Helpful Information
St Mary’s genomic sequencing lab can process 40, 000 tests per day. We have been at the forefront of genomic coronavirus lab testing during the pandemic and have undertaken over 650,000 Covid-19 PCR tests to help safeguard human health.
Genomic Covid-19 sequencing has been added to the travel rules to identify and prevent super spreading of Coronavirus variants. If our Coronafocus genomic sequencing lab test highlights a variant of concern, then individuals will receive a call from NHS Track and Trace, and their contacts will be asked to be tested.
Our Coronafocus Covid PCR lab test identifies the presence of the virus from an individuals’ swab sample. Genomic sequencing by our lab after identification of a Covid-positive result, maps the entire virus genetic code and identifies the specific variant. Covid-19 testing and sequencing needs to be completed within 72 hours from self-sampling by individuals.
Orders are dispatched in line with due dates under government rules, with next day delivery via DPD. Return the self-swabbed sample using the pre-paid Royal Mail 24-hour tracked envelope provided. Post the envelope in a Royal Mail priority post box located in every town. These are normal Royal mail postal boxes on the streets with a priority label on them https://www.royalmail.com/services-near-you#/ or on the Royal Mail app. Or you can hand deliver swab samples to St Mary’s, Suite 2, The Newnham Building, Chesterford Research Park, Little Chesterford CB10 1XL to avoid postal delays. You can arrange your own private courier to deliver the swab directly to our lab which is open 24 hours 7 days a week.
All orders placed directly with St Mary’s are dispatched the same day for next Working Day delivery AND in line with government rules relating to when kits can be supplied.
Please note government rules mean irrespective of when ordered you receive day 2 between day 0 and day 2 and the Test kit for day 2 and day 8 for Amber list Countries between day 0 and day 2 with two separate tests with unique barcodes for each day. Test to Release is dispatched separately on day 4 for arrival on Day 5 after you return to the UK. To avoid dispatch delays outside of our control please order at least 3 working days prior to required. Delays by Royal Mail return service are outside of our control. It is your responsibility to return the swab to us in sufficient time.
Multiple kit purchases for up to 10 people are available for booking’s on our website , apart from day2 & day8 travel packages, which need to be ordered separately for each individual traveller. For business or group requests greater than 10 people, please provide your contact details and send your requirements to sales@stmarysmedical.co.uk.
For queries relating to kit supply, delivery tracking, activation and results reporting for PCR testing purchased through a third party, please contact the company from whom you purchased your service for support and FAQ advice. We do not receive personal details for kits and testing services purchased through third parties. St Mary’s only supply lab testing services to third party companies accredited by UKAS and the DHSC. Third party service quality is monitored by UKAS and the DHSC to ensure it is in accordance with accreditation.
We do not accept cancelled tests. Booking a correct test can be done at covid.protected.com
A Coronafocus Test Kit is a product supplied by a health care professional and all orders are final when booked online. You have no statutory right to cancel your order or return your Coronafocus Test Kit. We reserve the right to cancel Coronafocus Test Kit orders and refund you the fee paid, should we have insufficient stock or be unable to deliver to the postal address provided in the online booking.
Check entry restrictions before you leave the UK as to whether a negative Fit to Fly/Travel COVID-19 PCR test is required to enter a country
For Green country arrivals into the UK, you need to take a pre-departure test and book prior to arrival on Day 0 (the day of arrival into the UK) a PCR test for day 2. There will be no need to home-quarantine for 10 days unless you test positive.
For Amber country arrivals into the UK you will need to quarantine for 10 days at home, take a pre-departure test, and book prior to travel a PCR test on day 2 and day 8. You have the option of taking a Test to Release PCR test on day 5 or later to end self-isolation early.
For Red country arrivals into the UK, a 10-day isolation in a quarantine hotel is mandatory, pre-departure testing and booking PCR testing must be done via the Government NHS provider. There is no Test to Release option.
PCR test results testing as positive are provided to NHS Track and Trace for variant surveillance and 10 days quarantine is required
- You must self-isolate on arrival in England as per Government guidelines for the full 10 days before you leave isolation.
- You can choose to take an additional Test to Release Covid-19 PCR test after 5 full days of self-isolation.
- Example: You leave a country on Monday morning and arrive in England on Monday afternoon. Tuesday will be your first full day of self-isolation. You can take a test no earlier than your 5th full day of self-isolation – Saturday. You must continue to self-isolate while you await your test results.
- If it is negative, you can end your quarantine.
- You will still be required to undertake the day 8 test even though not in quarantine.
- If testing is positive on day 5 or day 8, a further 10 day’s quarantine is required following the date of the positive Covid-19 PCR test.
- International travellers from red list’ countries are unable to opt-in to Test to Release, if arriving within 10 days of visiting a red-list country.
Everyone permitted to enter the UK must have proof of a negative PCR test prior to travelling to the UK – including UK citizens. The test must be taken in the 3 days prior to departure to the UK. In addition, everyone permitted to enter the UK must take additional Covid-19 tests on arrival in the UK while in quarantine:
Government rules require completion of a Passenger Locator Form detailing where you will quarantine on arrival into the UK and the booking reference number of the travel test you have taken. This is made easy when buying a Coronafocus test for submission of testing proof to the airline, ferry, or train operator you travel with.
The Process

Complete form, payment and customer dashboard
Complete the order form, make payment online and receive customer dashboard registration login instructions electronically.

Receive the self-test kit
The self-test kit, including instructions on how to carry out the test arrives at the address provided. Activate the test in the customer dashboard with swab taken date.

Send the Package
Send the self-sampled kits to arrive within 48hrs of sample swabbing to the lab using the pre-paid envelope provided

Access Results and Certificates
You receive notification that results are available in the customer dashboard < 24h of receipt of activated date stamped swab kit at the lab

Genomic Sequencing
Genomic sequencing results that tested positive from swabs taken on day 2, will be provided to NHS track and Trace for variant surveillance.

Platform for businesses
Manage your workplace Covid-19
tests from our online dashboard
St Mary’s provide Coronafocus@ Covid-19 PCR test kits to control the spread of Coronavirus variants in offices, factories, shops, schools, universities , aviation, rail, and bus transport.

Employee/Customer tests management
You can easily import and manage the assignments and testing data of your employees and customers.

Reports and certificates download
Access, print reports and flight certificates unique per passenger directly from your PC or smartphone.


Business & Industries
Offices, factories, media industry, oil and gas industry, mining.

Healthcare Providers
Care homes, GP surgeries,dental practices.

Transport & Logistics
Aviation, shipping, rail travel.

Educational Institutions
Schools, universities, technical colleges.
St Mary’s®, the world-leading cancer medicine and Covid-19 genomic sequencing laboratory is based in Chesterford Research Park, Cambridge, UK.
St Mary’s established in 2014, provides personalized precision medicine testing and St Mary’s genetic diagnostics to aid cancer patient therapy.
Using specific molecular targets and complex molecular platforms, the company is US CLIA certified and UK government approved to undertake Oncofocus® molecular testing of specific tumour gene mutations.
We utilise these platforms to perform high throughput genomic Covid-19 testing. Our laboratory services are key to alerting health professionals and protecting communities from variants travelling around the world.